About us
CelloVet is your supplier of real extruded regenerated cellulose which is also known as Cellophane for use in banding portosystemic shunts.
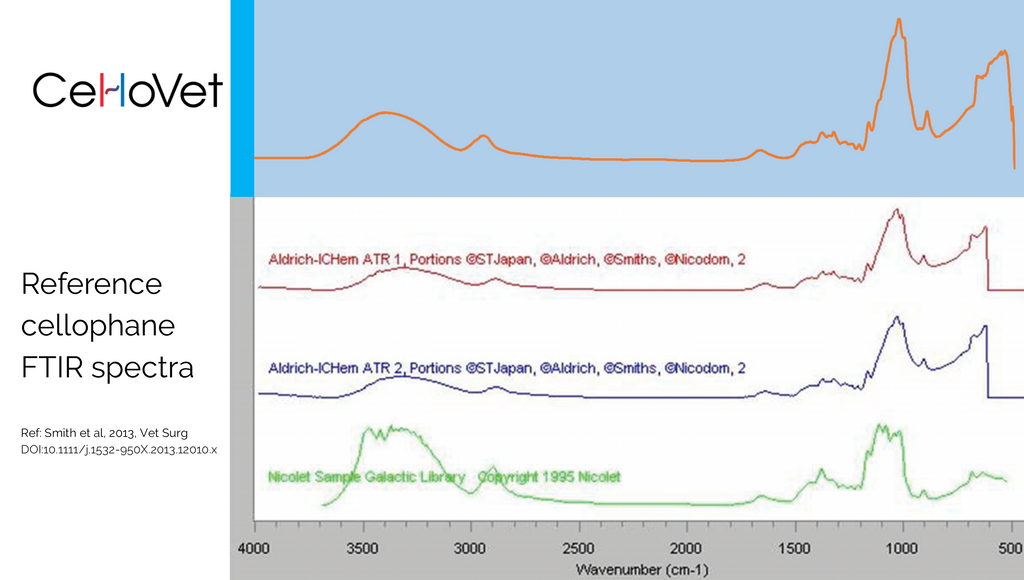
Based on the work by Smith et al (Veterinary Surgery, 2013) and our own personal experience in trying to find 'real' cellophane for use by veterinary surgeons, we founded CelloVet with the aim of removing the doubt about your implant's constituents and material properties. We went ahead and submitted our cellophane for Fourier Transform Infrared Spectroscopy as performed by Smith et al, and unsurprisingly, our results confirmed that CelloVet is consistent with real cellophane. Now you can rest easy knowing that your cellophane implant is cellulose and what to expect from it.
We guarantee they are all 100% extruded regenerated cellulose thin films, also known as Cellophane. Don't be fooled - cigarette packaging, Cellotape and other 'Cellophanes' are often coated with adhesives, chemically treated or are plastics made from oil.
As recommended by Smith et al, all Cellophane strips provided are aligned with the grain of the fibers. Furthermore, all products are shipped in NON-STERILE airtight, light reflective and moisture resistant bags to reduce spoilage. Shelf life of 6 months is expected when kept in a cool, dry and dark environment.
Our products are shipped NON-STERILE. You must remove the strips from the bag they were supplied in and place them, respectively, into individual sealable surgical instrument pouches for sterilization.
Autoclaving the strips in their surgical instrument pouches should be performed in accordance with you practice's protocols. Other methods of sterilization have been shown to reduce mechanical strength of the product by Smith et al (Veterinary Surgery, 2013).
To increase ease of handling during surgery and to maximize surface area for fibrosis around the shunt we recommend rolling or folding the strips lengthwise, parallel to their fiber grain. Once you have passed the product around the portosystemic shunt, apply at least 4 metallic vascular clips in an alternating pattern across both free ends to clamp it in place. Trim any excess product and remove from patient.
Intended for single use only in dogs and cats with portosystemic shunts.
Do not re-sterilize or reuse.
All products are made in the United States and shipped world wide. Local distributors can be found here.
